Twin study sheds light on how mitochondria influence gene regulation behind obesity
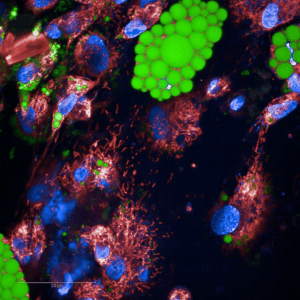
Microscopic image of differentiating human adipocytes grown in a cell culture. Mitochondria connected to each other are shown in red, nuclei in blue, and lipid droplets in the adipocytes in green. Image by Sini Heinonen.
A new twin study suggests that reduced mitochondrial metabolism can contribute to body fat accumulation and diminished insulin sensitivity which can, in turn, trigger chemical DNA modifications that alter gene regulation. The findings open new avenues for treating obesity by targeting mitochondrial metabolism or epigenetic regulation.
Mitochondria—the cell’s “power plants”—play a central role in energy metabolism and overall health. In obesity and its comorbidities, mitochondrial activity is often impaired, yet it has remained unclear whether this impairment increases the risk of obesity or whether obesity itself damages mitochondria.
Led by Miina Ollikainen, researchers at the Minerva Foundation Institute for Medical Research and the University of Helsinki untangled these complex cause-and-effect relationships by studying Finnish twins. Their results strengthen the view that a reduction in mitochondrial quantity helps drive the development of obesity.
Published in Nature Communications, the study involved nearly 90 Finnish twin pairs. Because twins share the same genetic background, researchers can disentangle genetic and environmental influences more precisely than in most other study designs and can use this similarity to model causal relationships statistically.
“Obesity is a major and growing public-health challenge in Finland. Our findings add key insight into how declining mitochondrial metabolism contributes to obesity and likely sets up a vicious cycle that sustains excess weight and hinders weight loss,” says group leader Miina Ollikainen.
When the team compared mitochondrial quantity with changes in DNA methylation, a chemical modification that regulate gene activity, one gene involved in cell growth and nutrient sensing stood out. The more overweight a person was—and the fewer mitochondria detected in their adipose tissue—the more active the gene SH3BP4 was.
“Our data suggest that when mitochondrial metabolism falters, for example because of calorie surplus, a feedback loop promoting obesity is set in motion, which in turn may activate SH3BP4,” explains first author and doctoral researcher Aino Heikkinen of the Minerva Foundation Institute for Medical Research and the University of Helsinki.
Because obesity can be assessed with many different metrics, comparing results from different studies is often difficult.
“Here we could show that especially reduced insulin sensitivity and an elevated body fat percentage—rather than weight gain alone—link both to mitochondrial quantity and to gene regulation,” the authors note.
Developing personalised obesity therapies requires an in-depth understanding of many intertwined mechanisms; this work marks a step toward that goal.
Original publication
Heikkinen A, Esser VFC, Lee SHT. et al. “Twin pair analysis uncovers links between DNA methylation, mitochondrial DNA quantity and obesity.” Nature Communications 16, 4374 (2025). https://doi.org/10.1038/s41467-025-59576-7
Further information
Miina Ollikainen
Minerva Foundation Institute for Medical Research, Helsinki
Institute for Molecular Medicine Finland (FIMM), HiLIFE, University of Helsinki
miina.ollikainen@helsinki.fi | +358 50 415 1276
Aino Heikkinen
Minerva Foundation Institute for Medical Research, Helsinki
Institute for Molecular Medicine Finland (FIMM), HiLIFE, University of Helsinki
aino.heikkinen@helsinki.fi